Which Best Describes an Oxidizing Agent
The oxidation state of the substance gets reduced during reduction. Cl2aq 2Braq 2Claq Br2aq Bromine Br is the oxidizing agent because it gains an electron.
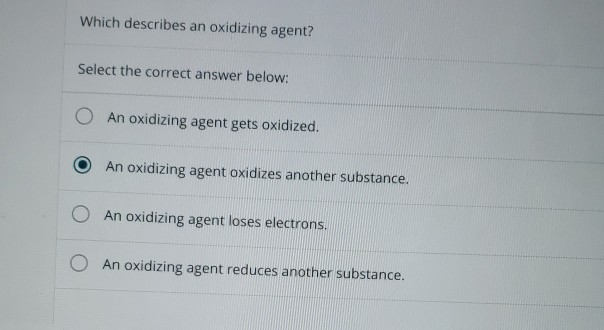
Solved Which Describes An Oxidizing Agent Select The Chegg Com
6 Which change is an oxidation.

. An oxidizing agent causes another. Answer not in Detail. The substance M which itself gets oxidized reduces other and is called as reducing agent.
An example of a good oxidizing agent is an alkali metal such as Na. Which of the following statements best describes an oxidizing agent. Question 7 Which of the following best describes an oxidizing agent.
Which best describes the oxidizing agent in this reaction. Chlorine Cl is the oxidizing agent because it gains an electron. Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron.
The oxidizing agent typically takes these electrons for itself thus gaining electrons and being reduced. D A substance that reacts with oxygen. Which best describes the oxidizing agent in this reaction.
Which metal cation is the best oxidizing agent. Reduction reaction is defined as the reaction in which a substance gains electrons. In the given reaction Oxidation state of Al changes from 0 to 3 therefore it is reducing agent.
The correct answer was given. 9 Which change always occurs when a metal atom is oxidised. 7 Which change in oxidation number indicates oxidation.
C O2 -. An oxidizing agent is a specie which accepts electrons and gets reduced in a chemical reaction. Which of the following statements best describes an oxidizing agent.
What is an Oxidizing Agent. B A substance that increases the number of oxygen atoms bonded. CO2In this case oxygen would be the oxidizing agent as it.
0 0 0 0 Choose An Option That Best Describes Your Problem. The gainof oxygen loss of hydrogen or loss of electrons. C A substance that is oxidized and.
Bromine Br is the oxidizing agent because it loses an electron. An oxidizing agent often referred to as an oxidizer or an oxidant is a chemical species that tends to oxidize other substances ie. Oxidizing agents are also known as oxidants or oxidizers.
Common examples of oxidizing agents include halogens such as chlorine and fluorine oxygen and hydrogen peroxide H 2 O 2. A good oxidizing agent is a metal in a high oxidation state such as Mn7 Oe. A A substance that is reduced and causes oxidation of another substanceb A substance that increases the number of oxygen atoms bonded.
Which best describes the oxidizing agent in this reaction. Oxidizing is defined as. Whereas oxidation state of Br is changing from -2 to -3 this means Br is gaining electrons therefore it is an oxidizing agent.
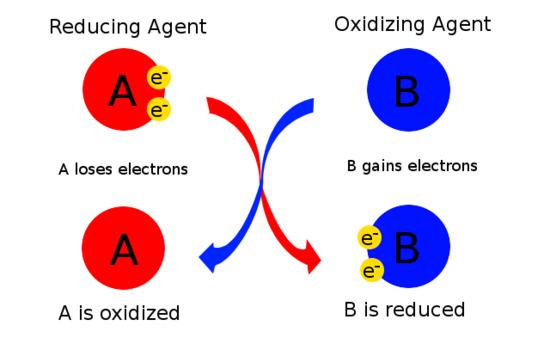
An oxidizing agent is a reactant that removes electrons from other reactants during a redox reaction. C A substance that is oxidized and causes reduction of another substance. A good oxidizing agent has the highest reduction potential.
The substance N which itself gets reduced oxidizes other and is called as oxidizing agent. An oxidizing agent loses electrons. Pb 2 Cr 3 Fe 2 Sn 2 Answer.
The strongest oxidizing agent in the list is F_2 followed by H_2O_2 and so on down to the weakest oxidizing agent Li. A A substance that is reduced and causes oxidation of another substance. 5 Which best describes the oxidizing agent in this reaction.
8 Which change in oxidation number represents oxidation. In chemistry an oxidizing agent oxidant oxidizer or oxidising agent oxidiser is a substance that has the ability to oxidize other substances in other words to accept their electrons. Cause an increase in the oxidation state of the substance by making it lose electrons.
Pb 2 Reason. Answer not in Detail. An oxidizing agent is thus an electron acceptor.
Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron. The oxidation number of an oxidizing agent increases. A compound is a molecule made of atoms from different elements.

Solved Question 7 Which Of The Following Best Describes An Chegg Com

Which Substance Is The Oxidizing Agent In This Reaction 2cuo C 2cu Co2 In 2022 Oxidizing Agent Substances Agents
No comments for "Which Best Describes an Oxidizing Agent"
Post a Comment